For Clinicians
Flu surveillance in California typically starts in September and goes through May of the following year with the most active months typically beginning in December and ending in April.
Mandatory Influenza Vaccination or Masking of Health Care Workers During Influenza Season
Health Officers for Alameda County and the City of Berkeley are re-issuing their joint Health Officer order (originally dated Aug. 28, 2013) mandating that all licensed health care facilities in Alameda County and the City of Berkeley require their health care workers (HCWs) to receive an annual influenza vaccination or, if they decline, to wear a mask during every influenza season while working in patient care areas. Influenza season dates are defined as November 1 to April 30 of the following year. This order is ongoing and applies to each influenza season, unless rescinded or modified.
- Joint Health Officer Order No. 20-16b November 2, 2020: Mandatory Flu Shots for Health Care Workers Order
- Health Advisory September 2018: Mandatory Influenza Vaccination or Masking of HCWs
- Health Advisory August 23, 2013: Mandatory Influenza Vaccination or Masking of HCWs
We have a mandatory masking order in place, however, flu vaccination coverage among Health Care Workers in Alameda County remains below the national Healthy People 2021 target of 90%.
- In 2016-17 Alameda County mean vaccination rate was 87% for all 14 facilities combined.
- In 2017-18 Alameda County mean vaccination rate was 84% for all 14 facilities combined.
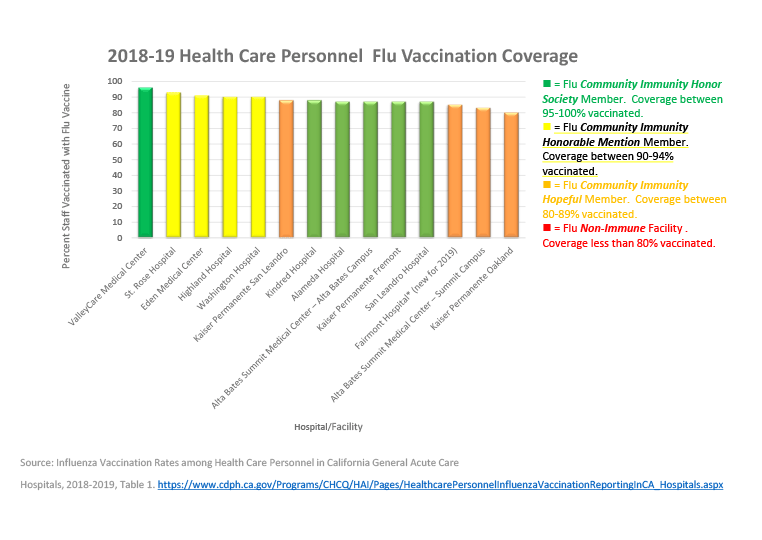
- In 2018-19 Alameda County mean vaccination rate for combined facilities increased to 87% of personnel vaccinated, with 9 out of 14 facilities below the Healthy People 2020 goal of 90% coverage rate.
The 2018-19 Health Care Personnel Flu Vaccination Coverage graph below depicts hospital ratings.
Additional Information
- Seasonal Influenza Information from the CDC
- CDC’s toolkits to improve health care personnel influenza vaccination rates
- Healthcare Personnel Influenza Vaccination in California Hospitals, 2017-18
Influenza Vaccine Composition for 2021-2022
Influenza vaccines expected to be available in the United States for the 2021–22 season will be quadrivalent vaccines.
Egg-based Vaccines
- an A/Victoria/2570/2019 (H1N1)pdm09-like virus;
- an A/Cambodia/e0826360/2020 (H3N2)-like virus;
- a B/Washington/02/2019 (B/Victoria lineage)-like virus; and
- a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus
Cell- or recombinant-based Vaccines
- an A/Wisconsin/588/2019 (H1N1)pdm09-like virus;
- an A/Cambodia/e0826360/2020 (H3N2)-like virus;\
- a B/Washington/02/2019 (B/Victoria lineage)-like virus; and
- a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus
As these changes occur and new vaccines become available, they will be reflected online: https://www.cdc.gov/mmwr/volumes/70/rr/rr7005a1.htm#influenzavaccinecompositionandavailablevaccines
Prevention
Vaccinate! Both intranasal and different injectable formulations are available. Encourage patients to get their flu vaccine. Refer to Vaccine Information for additional information on vaccine composition, dosing, storing, and administration or the comprehensive Advisory Committee on Immunization Practices (ACIP) guidelines, Prevention and Control of Influenza with Vaccines for the 2021-22 season.
Flu Vaccine from ACPHD: Each year, we distribute flu vaccine to providers who agree to certain guidelines about to whom they will administer the vaccine and how and when to report usage. Visit our Flu Vaccine Distribution Program page for more information.
Treatment
Early antiviral treatment is recommended for any person who has severe illness, is at high risk for severe illness or complication, or is hospitalized for influenza. It is ideal to start treatment with oseltamivir or zanimivir within 48 hours of onset, but may still be of benefit up to 4 to 5 days after onset. If you suspect influenza in a patient, don’t wait for lab results to begin treatment.
For more information refer to the Centers for Disease Control and Prevention (CDC): Influenza Antiviral Medications: Summary for Clinicians.
Pregnant and Postpartum Women
Pregnant and postpartum women are at high risk for serious complications from influenza virus. Health care providers should encourage all women who might be pregnant, who are pregnant and who are postpartum to get vaccinated. Influenza vaccination can be administered at any time during pregnancy, before and during the influenza season. Influenza vaccination can also help protect against premature labor and delivery. Antivirals are recommended for treating influenza in pregnant women. For more information and an algorithm for dosing in this age group please refer to the ACIP guidelines.
Influenza Vaccine Dosage
Children aged 6 months through 8 years who are receiving influenza vaccine for the first time, and some in this age group who have previously been vaccinated, require two doses of vaccine administered ≥4 weeks apart. Refer to Vaccine Information for additional information on vaccine composition, dosing, storing, and administration or the comprehensive Advisory Committee on Immunization Practices (ACIP) guidelines. For more information and an algorithm for dosing in this age group please refer to the ACIP guidelines.
Influenza Vaccination of Persons with a History of Egg Allergy
Persons with a history of egg allergy who have experienced only hives after exposure to egg should receive influenza vaccine. The ACIP Guidelines recommend watching these and non-allergic persons for 15 minutes following vaccination to decrease the risk of injury due to syncope. Persons with severe egg allergy should be vaccinated under the supervision of a medical provider who is able to recognize and manage severe allergic conditions.
Laboratory Specimens
When submitting individual specimens for influenza testing to the Alameda county public health lab, please use this form:
Infection Control
Clinics: Please provide masks and hand sanitizer for patients with suspected influenza and ask them to mask before sitting in waiting rooms.
Inpatient: Standard and droplet precautions should be implemented for patients with suspected or confirmed influenza, including placement in a private room and placing a surgical mask on patients if they must be transported to other areas of the facility.
- CDPH Guidance for Influenza Prevention in Health Care Settings (This 2010 document is still in effect.)